Electrochemical CO2 Reduction at Membrane Electrode Assemblies Operated at Elevated Temperatures
Electrochemical CO2 reduction is an electrolysis process that can transform excess CO2 into valuable chemicals, thus contributing to closing the carbon cycle, for example, by producing carbon monoxide on silver catalysts.
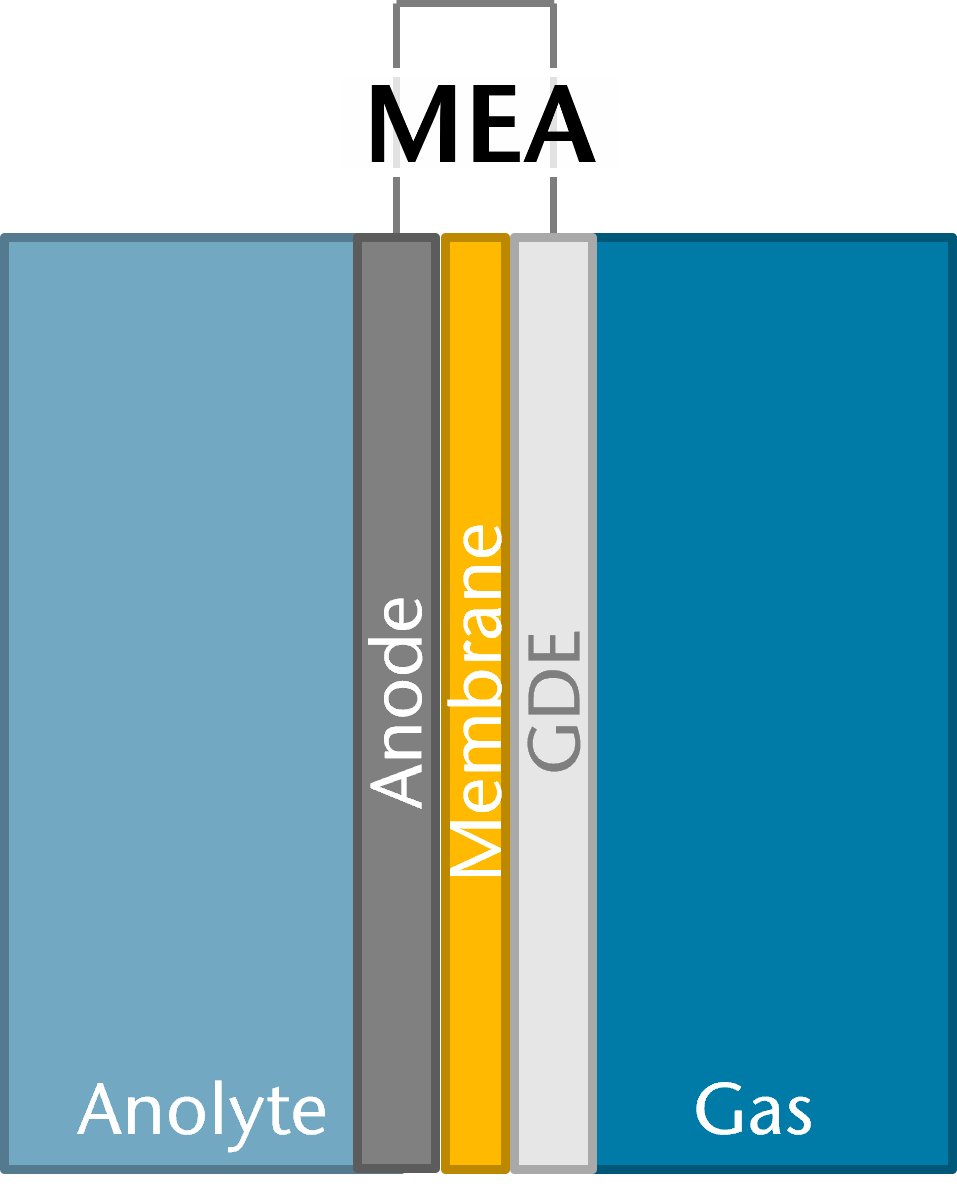
A promising setup for this process includes membrane electrode assemblies (MEAs), which enhance energy efficiency by avoiding electrolyte gaps between electrodes. Currently, tests of MEAs developed at the institute are predominantly performed at room temperature. However, even with unheated electrolyte and gas feeds, temperature effects will become important on industrial scale. As this will undoubtedly affect the kinetics, mass transport and other phenomena within the electrolyzer, a detailed analysis on the influence of operating temperature on the performance is required. At the same time, it is to be expected that the test procedure and/or experimental assembly will have to be partly adapted to produce reliable results.
Therefore, the aim of this thesis is to provide a systematic experimental analysis on the above-mentioned aspects. Extensive prior knowledge in electrochemical process engineering is not necessary.
Information about the thesis
Type of work: Master's thesis
Start of work: by arrangement
Working method: experimental
Place of work: ICVT, Clausthal
Contact person: Lydia Weseler
Information about the thesis as PDF