Investigation of measures to avoid stray currents in alkaline water electrolysis
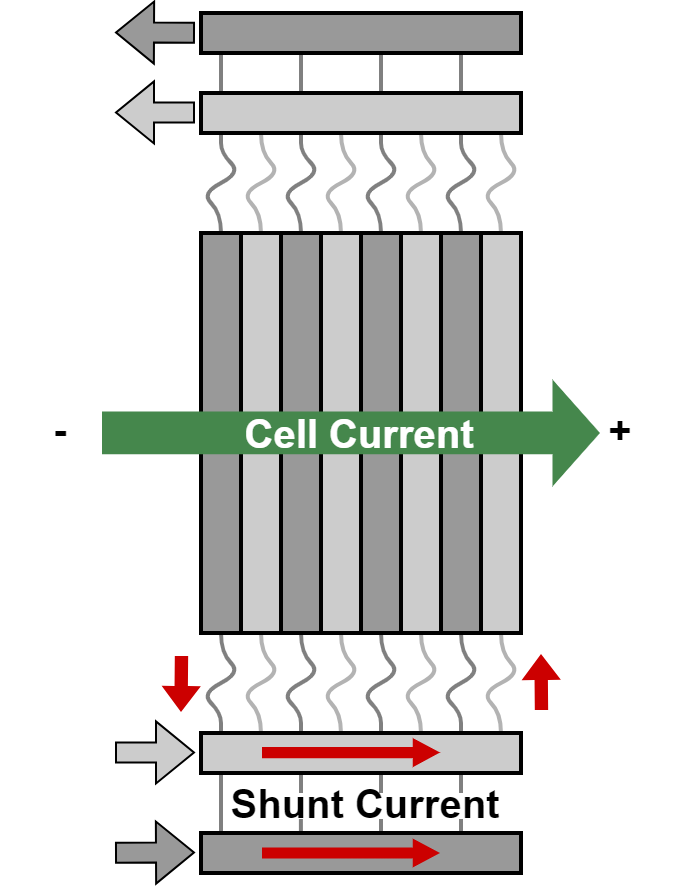
Hydrogen (H2) is an important raw material for the chemical industry and will be used in the future to store and transport renewable energy. Alkaline water electrolysis, which uses a highly concentrated potassium hydroxide solution as the electrolyte for the electrochemical cell, is a promising process for the industrial production of H2 from renewable electricity. To scale up water electrolysis as an industrial process, several individual cells are stacked on top of each other to form a 'stack' and supplied by a common electrical current. The individual cell voltages add up to a total voltage in the range of 100-600 V for the entire stack. If all the cells are supplied together from a single electrolyte circulation system, current may also flow through the conductive electrolyte. This causes a short-circuit current to flow past the actual cells, which is a phenomenon known as stray currents. These reduce the efficiency of hydrogen production and lead to corrosion in other components.
This work will investigate various measures to reduce stray currents that have potential for industrial application. This can be done by selectively interrupting or restricting the flow of liquid so as to increase the resistance in the lye. Possible methods include the addition of gases, the incorporation of drip edges or interruption by moving components. As part of the thesis, these methods will be researched, experimentally tested and evaluated in terms of their feasibility, implementation costs and stray current avoidance efficiency in industrial applications.
Information about the thesis
Type of thesis: Bachelor's thesis / Master's thesis
Start of work: November 2024, by arrangement
Working method: experimental
Place of work: EST, Goslar
Contact person: Simon Appelhaus
Information about the thesis as PDF