Electrochemical CO2 Reduction at Bipolar Membrane Electrode Assemblies
Electrochemical CO2 reduction is an electrolysis process that can transform excess CO2 into valuable chemicals, thus contributing to closing the carbon cycle, for example, by producing carbon monoxide on silver catalysts.
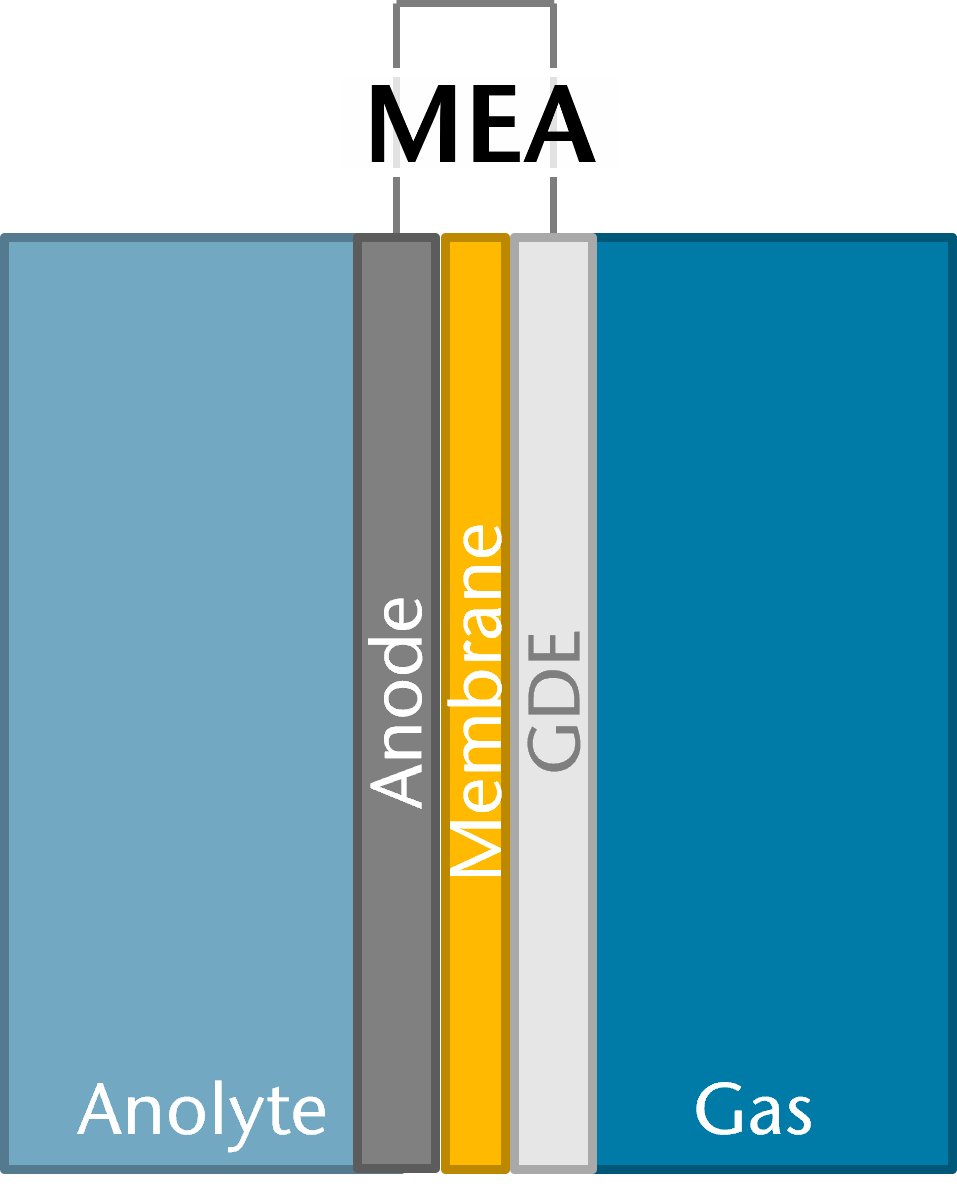
A promising setup for this process includes membrane electrode assemblies (MEAs), which enhance energy efficiency by avoiding electrolyte gaps between electrodes. Currently, anion exchange membranes are predominantly used, though they pose challenges in MEA configurations, such as increased precipitation of carbonate or bicarbonate salts, which serve as electrolytes, at the cathode (gas diffusion electrode, GDE). Since this side of the electrolysis cell is supplied only with gas, these salts cannot be removed and obstruct active sites where the reaction takes place. Bipolar membranes can help to circumvent this issue.
The goal of this thesis is to successfully produce a bipolar membrane, implement it in a MEA, and conduct subsequent testing in an existing experimental setup. Extensive prior knowledge in electrochemical process engineering is not required.
Information about the thesis
Type of work: Master's thesis
Start of work: by arrangement
Working method: experimental
Place of work: ICVT, Clausthal
Contact person: Lydia Weseler
Information about the thesis as PDF